 Research Article
Research Article
First Report of the Green Seaweed Codium bursa (Olivi) C. Agardh from Saint Martin’s Island
Mohammad Khairul Alam Sobuj1*, Md. Mohidul Islam1, Shafiqur Rahman1 and Yahia Mahmud2
1Marine Fisheries and Technology Station, Bangladesh Fisheries Research Institute, Cox’s Bazar-4700, Bangladesh
2Bangladesh Fisheries Research Institute, Mymensingh-2201, Bangladesh
Mohammad Khairul Alam Sobuj, Marine Fisheries and Technology Station, Bangladesh Fisheries Research Institute, Bangladesh.
Received Date:January 17, 2023; Published Date:April 11, 2023
Abstract
The diversity of seaweed in Bangladesh territory is moderately high, with 154 recorded species of all classes. A detailed inventory of the available seaweed species is still ongoing and has a shortage of these seaweed species’ biological and ecological statistics. We highlight the records of the green algae Codium bursa found on the rocky shore of Saint Martin’s Island, which is the first species documented in the subcontinental region. We also provide detailed taxonomic and ecological data for further comparisons. Our new finding focuses on the need for a more detailed inventory, investigation and analysis in-depth for the available seaweed species obtainable in the coastal area of Bangladesh.
Keywords:Algae; Bangladesh; Chlorophyta; Codium bursa; Phycology.
Introduction
Seaweed is a large group of macroscopic marine algae which are generally found in three major groups, i.e., Chlorophyta (green seaweed), Phaeophyta (brown seaweed) and Rhodophyta (red seaweed). They are a decent potential source of diverse bioactive compounds and natural antioxidant activity and have acquired much attention [1-2]. The most promising application of seaweeds is found in food, animal feed, fertilizer, biofuel production, bioremediation, pharmaceutical, wastewater treatment and cosmetic industry [3-4]. A significant number of seaweeds are found along Bangladesh’s 710-kilometer coastline and in the Bay of Bengal in the Indian Ocean. About 165 seaweed species have been identified along Bangladesh’s coastline, belonging to 77 genera comprising 38 Chlorophyta, 5 Chrysophyta, 27 Cyanophyta, 46 Phaeophyta and 49 Rhodophyta [5]. Other scholar [6] documented about 193 species of marine algae from Bangladesh and classified them into Chloro phyta (51 spp), Phaeophyta (54 spp) and Rhodophyta (88 spp). A more extensive work was done by Islam et al. [7] with 134 individual seaweeds photograph, their abundance, distribution and taxonomic list, where most of the seaweed was identified from Saint Martin’s Island. Our present study adds C. bursa, a species previously unrecorded from Bangladesh.
The biofunctional activity of C. bursa was less described in the literature. However, some extracts of this seaweed showed relatively good antifungal activity, especially against Penicillium expansum. The most dominant fatty acids in C. bursa were palmitic, oleic, linoleic and stearic. Also, some volatile oil and other vital compounds, such as heptadecane and docosane, are present in fresh C. bursa [8]. Guiry and Guiry [9] described 290 species of Codium that are now widely accepted. Species of Codium may be managed with supports to rocks or hard shells and form small colonies. During an inven tory survey on Saint Martin’s Island in Southeastern Bangladesh, a surprising Codium species was collected, attaching the dead coral reef new to Bangladesh’s marine algal flora. The specimen was identified as Codium bursa (Olivi) C. Agardh, which has not yet been identified from the vicinity of the Bay of Bengal. Moreover, our results are the first time a species has been documented anywhere in the subcontinental region. Moreover, this is the second species recorded in Bangladesh in the genus Codium soon after C. fragile. This article provides a detailed morphological description of this species with its distributions, habitat and biology.
Materials and Methods
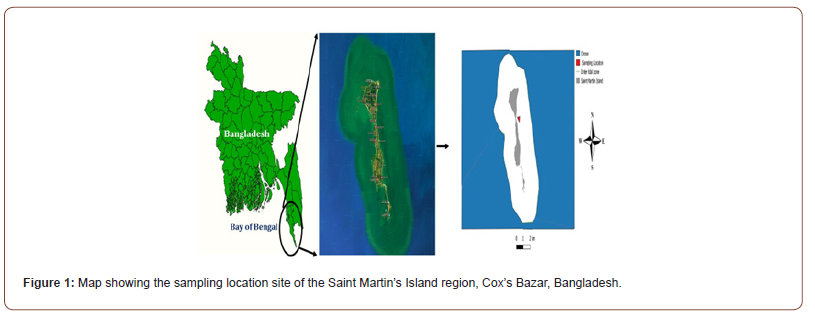
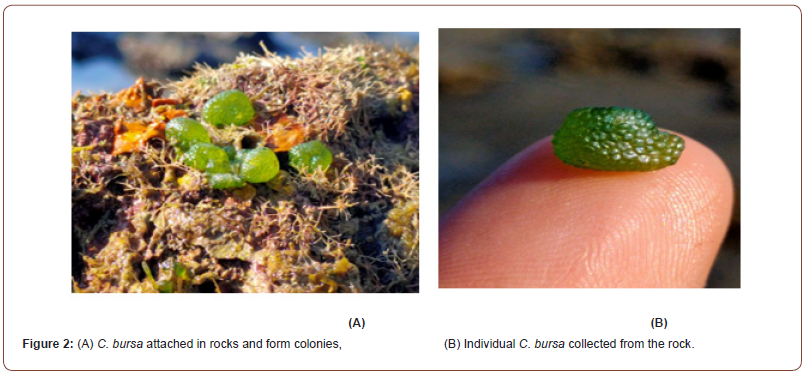
While conducting a biodiversity survey focusing on the identification of new seaweed species in the northeastern part of the Bay of Bengal, the specimen was encountered on 08 December 2021 from the rocky shore of Saint Martin’s Island (92°19ʹ41.716ʺE and 20°37ʹ2.54ʺN, Figure 1). The specimen was photographed and identified as a Chlorophyta, C. bursa (Olivi) C. Agardh, 1817 (Figure 2). These algae were the first recorded in Bangladesh at Saint Martin’s Island. Specimens were rubbed from the dead coral reef with a small sharp knife, and field preservation was subsequently performed with alcohol. Some seaweed samples were also inserted and pressed between herbarium paper sheets and left to dry in the sun for the complete removal of moisture. Samples were then brought to the Marine Fisheries and Technology Station, Bangladesh Fisheries Research Institute, Cox’s Bazar, Bangladesh, for further investigation. For proper identification, the specimen’s external morphology and internal anatomy were observed and matched with the described images [9]. All sections were made manually with double-sided razor blades. Unstained sections were used to make fresh mounts, which were then looked at with a compound light microscope (Leica DM500). On the field, seaweed photos were captured by a digital camera (Sony DSC-H300). Voucher specimens were also preserved at BFRI herbarium. To adjust the brightness, contrast and resolution of the image Adobe Photoshop 7.0 was used.
Results and Discussions
Taxonomic classification
a. Kingdom: Plantae
b. Subkingdom: Viridiplantae
c. Phylum: Chlorophyta
d. Subphylum: Chlorophytina
e. Class: Ulvophyceae
f. Order: Bryopsidales
g. Family: Codiaceae
h. Genus: Codium
i. Species: Codium bursa
Distribution:
These marine green algae are predominantly found in the Mediterranean Sea (especially near Spain) and the East Atlantic Ocean, wherein the latter is recorded from Portugal, England and Ireland (two locations Cos Donegal and Galway), south to Morocco and Canary Islands. It is common across the Mediterranean and often grows with the seagrass Posidonia oceanica. In certain areas, it also drifts among the leaves of Posidonia.
Habitat: C. bursa generally grows on rocky shores and forms a colony from upper to lower intertidal rock pool zones. They usually sublittoral attached to rock to 10-15 m deep.
Biology: In most cases, they form small colonies. The thallus fragments during reproduction, which occurs in the autumn. P. oceanica or rocky bottoms are where it is generally found. Surface depths of about 40 meters and well-lit depths of up to 90 meters are common. Storm waves have wiped out areas on the sea or the surface [10]. It was found that the life span of C. bursa sometimes surpasses 16 years (i.e., the number of years it took to attain its largest size) [11].
Morphological Description: C. bursa is a marine alga that can grow up to 30 cm in diameter. It usually appears as a spongy sphere of utricles with a cortex on the surface. It is composed of loosely packed filaments that create a vortex of single-celled bladder-like or club-shaped structures called utricles. It is dark green and velvety in color. A holdfast of filaments attaches the alga. They are generally grass green to blue-green and are 10-400 mm wide. When young, the velvety surface is hard, solid, and spherical; as it ages, it develops into undulating and converging growths. As it ages, it will become hollow and filled with water. Made up of minute filaments that tangle with one another, the ends of which form utricles that may be seen as tiny bumps on the surface with the aid of a hand lens. The spherical structures can detach and be carried ashore. They often have a bloated look and a slightly pleasurable oceanic odor (Figure 3).
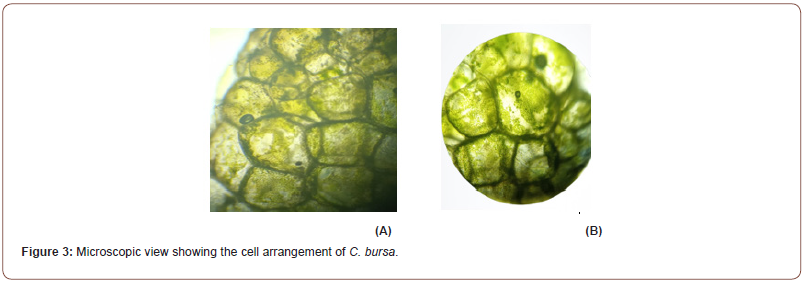
The chlorophyte C. bursa is exceptional among the multicellular macroalgae because it forms balloons having a sturdy algal wall containing a significant water mass. That is why it is also known as a green sponge ball or Ball alga. C. bursa is an ideal testing organism for demonstrating the value of thallus type and thickness as a limit to macroalgal physiological attributes due to its unique size and shape. C. bursa is a macroalga that is very dense. With growing size, the thallus wall thickened in small individuals from around 4 mm to about 6 mm in the most prominent individuals. It produces something like a green sponge in the shape of hollowed, sub-spherical cushions (the thallus). The thallus is generally available in sizes ranging from 8 cm to 40 cm. The thallus is almost perfectly spherical in its prime but flattens out and hollows out in the center. Touchable, hollow on the inside and conveniently compressible. The color ranges from light to dark green. The surface of a red thallus will also be covered with microscopic blue-green algae. Its texture resembles a sponge’s [10].
C. bursa occurs in a variety of sizes. Water may be scarce in small C. bursa individuals (if diameter = 2 thallus thickness). Because of their growing size, individuals tend to fill up with water as they attain more prominence. The thallus of alga that covers the balloon’s surface should grow according to the square of each individual’s size. In contrast, the amount of water within will grow as the diameter grows to the third power. As a result, the tissue’s relative contribution must decrease as its diameter decreases. The thalli possessed a spectral pigment concentration greater than required to absorb 90% of incoming sunlight. As expected for a thick plant, the amount of pigment per unit of weight was still low [12].
Their growth rates were relatively low (mean ± SE: 2.2 ± 0.4 10-3 d-1) and the doubling time was 1.24 ± 0.1 yr. Their growth rates were correlated strongly with the variations in external and internal nutrition concentrations that occur throughout the year, suggesting nutrients, most probable phosphorus, as a limiting factor [11]. The average concentration of phosphorus in the tissues of C. bursa was found to be C/P = 1472 ± 64 [13]. Because of their slow growth rate and low nutrient requirements, macroalgae are crucial components of oligotrophic coastal environments. They keep their losses to a minimum because of their high volume and lengthy lifespan [14]. Due to their slow-growing pattern, macroalgae are great examples of plants that can handle stress and are not too dependent on the availability of resources. Their yield is restricted not by the availability of resources but rather by their growth pattern [15]. In contrast, slow-growing macroalgae have thick thalli and low surface-to-volume ratios, which should limit their ability to absorb nutrients [16].
Conclusion
C. bursa (Olivi) C. Agardh, 1817 is a newly recorded green seaweed from Saint Martin’s Island, Bangladesh, and represents a new species record for the marine flora of Bangladesh. As Saint Martin’s Island is an ecologically diverse and unique area, the identification of C. bursa from the Saint Martin’s Island is not a surprising thing at all. This species is a holotype and is currently described for the first time from the subcontinental region. Further research could be performed on the phylogenetic status of this species with the available bioactive compounds.
Acknowledgement
This research was funded by Bangladesh Fisheries Research Institute (BFRI), Ministry of Fisheries and Livestock, Bangladesh.
Conflict of Interest
The authors declare no conflict of interests.
Author contributions statement
M.K.A.S. performed field work, specimen collection, data compilation. conceptualization, designed and drafted the original manuscript. M.M.I., S.R. and Y.M. performed supervision, editing and revising of the manuscript. All authors read and approved the final version of the manuscript.
References
- Sobuj MKA, Islam MA, Haque MA, Islam MM, Alam MJ, et al. (2021) Evaluation of bioactive chemical composition, phenolic, and antioxidant profiling of different crude extracts of Sargassum coriifolium and Hypnea pannosa J Food Meas Charact 15(2): 1653-1665.
- Sobuj MKA, Islam MA, Islam MS, Islam MM, Mahmud Y, et al. (2021) Effect of solvents on bioactive compounds and antioxidant activity of Padina tetrastromatica and Gracilaria tenuistipitata seaweeds collected from Bangladesh. Sci Rep 11(1): 1-13.
- Porse H, Rudolph B (2017) The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J Appl Phycol 29(5): 2187-2200.
- Pereira L, Gheda SF, Ribeiro Claro PJ (2013) Analysis by vibrational spectroscopy of seaweed polysaccharides with potential use in food, pharmaceutical, and cosmetic industries. Int J Carbohydr Chem 2013: 1-7.
- Islam NAKM (1976) Contribution to the study of the marine algae of Bangladesh. Bibl Phycol 19: 1-253.
- Sarkar MSI, Kamal M, Hasan MM, Hossain MI (2016) Present status of naturally occurring seaweed flora and their utilization in Bangladesh. Res Agric Livest Fish 3(1): 203-216.
- Islam MM, Hoq ME, Haque MA, Khan MSK, Hasan J, et al. (2019) Seaweeds of Bangladesh coast. Bangladesh Fisheries Research Institute, Mymensingh, Bangladesh.
- Jerkovic I, Kranjac M, Marijanovic Z, Sarkanj B, Cikos AM, et al. (2019) Chemical diversity of Codium bursa (Olivi) C. Agardh headspace compounds, volatiles, fatty acids and insight into its antifungal activity. Molecules 24(5): 842.
- Guiry MD, Guiry GM (2022) AlgaeBase. World-Wide Electronic Publication. National University of Ireland, Galway. Available online: (accessed on 22 November 2022).
- Guiry MD, Guiry GM, Morrison L, Rindi F, Miranda SV, et al. (2014) AlgaeBase: an on-line resource for algae. Cryptogamic Algologie 35(2): 105-115.
- Vidondo B, Duarte CM (1995) Seasonal growth of Codium bursa, a slow-growing Mediterranean macroalga: insitu experimental evidence of nutrient limitation. Mari Ecol Prog Ser 123: 185-191.
- Agusti S, Enriquez S, Frost Christensen H, Sand-Jensen K, Duarte, CM (1994) Light-harvesting among photosynthetic organisms. Funct Ecol 8(2): 273-279.
- Geertz Hansen O, Enriquez S, Duarte CM, Agusti S, Vaque D, et al. (1994) Functional implications of the form of Codium bursa, a balloon-like Mediterranean macroalga. Mari Ecol Prog Ser 108: 153-160.
- Duarte CM (1995) Submerged aquatic vegetation in relation to different nutrient regimes. Ophelia 41(1): 87-112.
- Grime JP (1974) Vegetation classification by reference to strategies. Nature 250(5461): 26-31.
- Hein M, Pedersen MF, Sand Jensen K (1995) Size-dependent nitrogen uptake in micro-and macroalgae. Mari Ecol Prog Ser 118(1): 247-253.
-
Mosha SS, Felix S, Manikandavelu D, Felix N, Samuel M T.L.S, Meenakshisundaram M. Effect of Dietary Mixture Containing Azolla and Spirulina Platensis on Physiological, Metabolic, Immunological and Histological Performance of GIFT-Tilapia (Oreochromis niloticus) Cultured in Lined Ponds. Ad Oceanogr & Marine Biol. 3(3): 2023. AOMB.MS.ID.000562.
-
Azolla, Spirulina platensis, GIFT tilapia, Digestive enzymes, Immunity, Carotenoid content, Isocaloric, Anisonitrogenous, Spirulina platensis, Dissolved Oxygen
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






